Neurosoft Bioelectronics sets record for most electrodes in a soft and stretchable brain interface tested in a human
Share this article
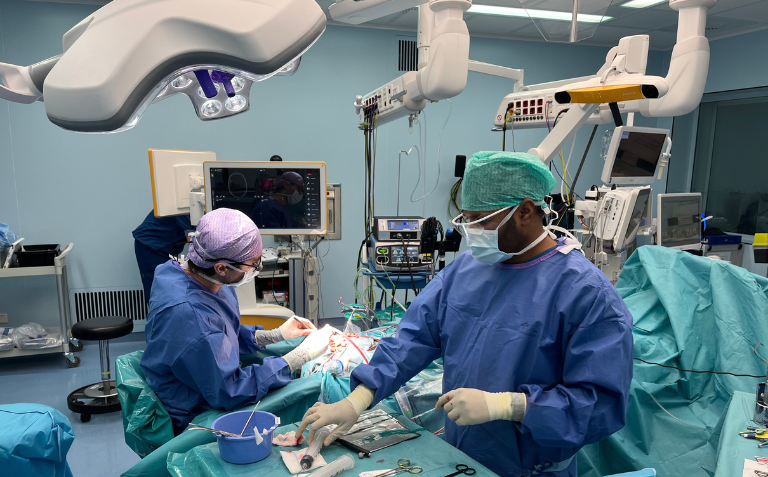
Neurosoft Bioelectronics SA, a Swiss neurotechnology startup based at Campus Biotech in Geneva, has achieved a major milestone with the successful testing of its high channel count brain interface in the first two human patients.
This milestone was reached in February 2025 at University Medical Center (UMC) Utrecht, where a team led by Professor Maeike Zijlmans employed the device during epilepsy surgery.
This novel brain interface is designed to detect key epilepsy biomarkers – high-frequency oscillations and fast ripples – allowing surgeons to identify epileptic tissue with unprecedented precision during the procedure.
With 64 sensing channels embedded in a stretchable material that is 1’000 to 100’000 times softer than conventional electrodes, the device conforms seamlessly to the brain’s surface. This softness improves comformability with the brain, while ensuring high-resolution neural recordings, helping neurosurgeons distinguish between healthy and epileptic tissue more accurately, thereby reducing the risk of either excessive or insufficient tissue removal.
“We are pushing the frontier of neurotechnology focusing on materials that are intended to interface seamlessly with the brain,” said Nicolas Vachicouras, CEO and co-founder of Neurosoft Bioelectronics. “This milestone solidifies our leadership in soft, stretchable brain interfaces and paves the way for broader applications in neuroprosthetics and brain-computer interfaces.”
“The Neurosoft flexible grid has been developed according to our clinical needs. The flexibility can be especially useful for recording small epileptic activity, like high frequency oscillations and for checking the surgical borders after initial tissue resection.” said Prof. Maeike Zijlmans, epilepsy specialist at UMC Utrecht.
These first two successful trials mark the beginning of a larger, 12-patient study at UMC Utrecht, designed to validate the technology for broader clinical use. The ultimate goal is to secure European regulatory approval and make this high-resolution technology available to epilepsy surgery centers across Europe.
The success of this initiative is the result of close collaboration between Neurosoft Bioelectronics and UMC Utrecht, demonstrating how startups and hospitals can work together to bring cutting-edge medical technologies into real-world clinical settings.
This work has been made possible through the support of the Wyss Center for Bio and Neuroengineering, which has played a key role in fostering Neurosoft Bioelectronics’ technological and clinical advancements. Additionally, the study conducted at UMC Utrecht is funded by the European Research Council (ERC), reflecting the project’s significance in advancing neurotechnology and epilepsy research.
Source: press release