FDA Clears Abionic’s IVD CAPSULE PSP for Early Detection of Sepsis
Share this article
Abionic, a Vaud-based medical diagnostics company, announced that its IVD CAPSULE PSP test received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This test, designed for the early detection of sepsis, represents a major breakthrough in sepsis diagnostics by significantly reducing the time-to-detection, potentially improving patient outcomes. The FDA clearance marks an important step for Abionic as it enters the U.S. market, following its EU IVDR certification in July 2022.
Sepsis: A Global Health Crisis
Sepsis is a life-threatening medical condition that occurs when the body has an extreme response to an infection. This condition is responsible for over 11 million deaths annually, accounting for 20% of all deaths worldwide. It is particularly prevalent in the United States, where it affects 1.7 million people and results in healthcare costs of $38 billion each year. Despite these alarming statistics, diagnosing sepsis early remains a significant challenge due to its non-specific symptoms, which often mimic those of other common illnesses.
The Sepsis Alliance notes that 80% of sepsis-related deaths could be prevented with timely detection and treatment. However, because sepsis often presents with general symptoms such as fever, confusion, or difficulty breathing, healthcare providers face difficulties in identifying it before it progresses to a life-threatening stage.
IVD CAPSULE PSP: An Early Warning System for Sepsis
The IVD CAPSULE PSP test aims to address these challenges by detecting sepsis earlier than current diagnostic methods. The test leverages Pancreatic Stone Protein (PSP), a biomarker produced by the pancreas and immune cells, which has shown promise as an early indicator of sepsis. PSP levels in the bloodstream increase in response to infection and inflammation, offering healthcare providers a valuable tool for sepsis detection up to 24 to 48 hours earlier than conventional diagnostic approaches.
Clinical studies have demonstrated that elevated PSP levels closely correlate with the onset and progression of sepsis. By using PSP as a biomarker, clinicians can monitor patients in critical care settings and activate sepsis treatment protocols sooner, significantly improving patient survival rates.
By reducing the time-to-detection, the IVD CAPSULE PSP test enables healthcare professionals to initiate treatment protocols earlier, which can be the difference between life and death for many patients. Sepsis is a condition that progresses rapidly, and early intervention is essential to improve patient outcomes and reduce mortality rates.
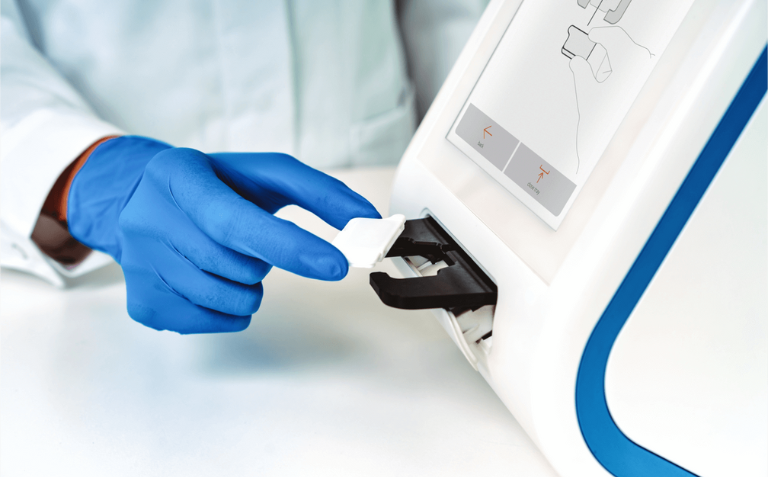
The abioSCOPE® Platform: Rapid Diagnostics at the Point of Care
Abionic’s IVD CAPSULE PSP test operates on the abioSCOPE® platform, a near-patient rapid diagnostics system. This award-winning platform uses nanofluidic technology to provide lab-quality results from just a single drop of blood within minutes. The abioSCOPE® integrates seamlessly into routine clinical workflows, delivering fast and accurate results that are critical for time-sensitive conditions like sepsis.
The ability to quickly measure PSP levels allows clinicians to make informed decisions about patient care much sooner than with traditional diagnostic methods. This is particularly important in intensive care units and emergency departments, where every minute counts in preventing sepsis from advancing to more severe stages such as septic shock, which carries a high risk of death.
FDA Clearance: A Milestone for Abionic’s U.S. Expansion
With FDA 510(k) clearance, Abionic is now positioned to offer its IVD CAPSULE PSP test to U.S. healthcare providers. This clearance not only validates the efficacy and safety of the test but also paves the way for its adoption in critical care settings across the country.
Patrick Pestalozzi, CEO of Abionic, emphasized the significance of this milestone, stating, “Achieving FDA 510(k) clearance for IVD CAPSULE PSP marks a significant step for Abionic and confirms our ability to meet the need for quick and reliable sepsis testing. This clearance will allow us to deploy our solutions across the United States and provide clinicians in acute care settings with a proven tool to accelerate the time-to-detection of sepsis.”
➡️ Source: Press Release