€2M Eurostars funding for a project led by Calciscon
Share this article
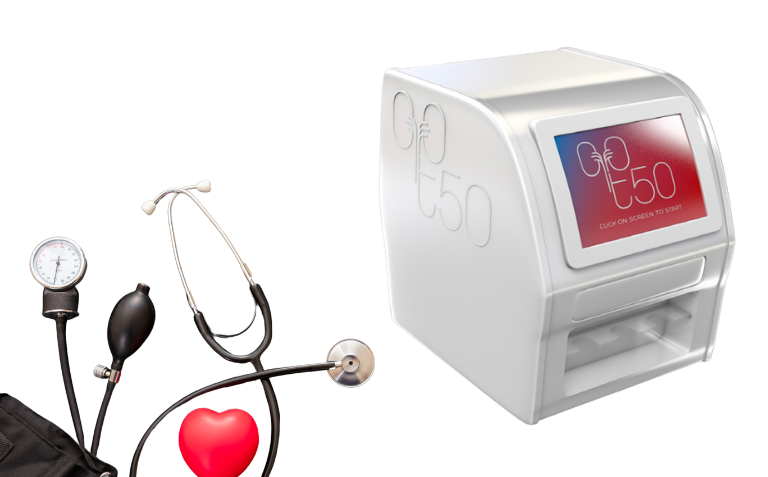
CALCISCON, AMSTERDAM UNIVERSITY MEDICAL CENTERS and ADMESY have signed a collaborative agreement for the development of a laboratory benchtop analyzer for the measurement of the T50 test.
T50 is a unique test for the measurement of calcification propensity in a blood sample. The primary use of T50 is to revolutionize the treatment of patients suffering from chronic kidney disease, particularly dialysis patients, with the potential to significantly reduce their cardiovascular burden. The collaboration partners combine excellence in calcification in vitro diagnostics (Calciscon), in-depth know-how on optical detection systems (Admesy) and world-renown expertise in clinical chemistry and nephrology research (Amsterdam UMC). The CALPROTECT consortium is supported by €2M Eurostars funding.
The consortium will develop a T50 analyzer to allow clinical laboratories worldwide to easily measure calcification propensity (T50) in blood. T50 measures the formation velocity of nanometer-sized calciprotein particles (CPP), known to induce calcification, inflammation and oxidative stress.
“Having the availability of T50 testing in clinical practice will improve identifying those people treated by dialysis at highest risk, while its serial measurements makes it possible to personalise treatment and holds promise to improve their outcomes”, said Prof. Dr. Marc Vervloet (Nephrologist at Amsterdam UMC). Admesy will develop a novel optical detection strategy for detection of the calcified nanoparticles.
“The challenges associated with creating a robust, reliable and economical detection system for the minuscule CPP fall exactly into our R&D strong-hold in high-performance optical systems” commented Jimmy van den Bergh (R&D Manager at Admesy). Calciscon will lead the integration of the technology into an IVD-grade analyzer, that will be tested in clinical settings at Amsterdam UMC.
“Our hospital clinical laboratory is uniquely suited to assess the performance of a device when used with patients at high risk for vascular calcification”, said Dr. Henrike Hamer (Clinical Chemist at Amsterdam UMC).
Calciscon is preparing for the launch of T50 for use with chronic kidney disease patients, particularly dialysis patients through strategic partnerships in Europe, USA and the Far East. “This exciting development project is instrumental for bringing T50 to treating physicians worldwide to improve patient care” said Vincent Linder Ph.D. (CEO at Calciscon)
Source: Press release