Posts Tagged ‘spinal cord injury’
ONWARD Medical Secures FDA Approval for First Non-Invasive Spinal Cord Stimulation System
ONWARD Medical has reached a significant milestone in spinal cord injury (SCI) treatment, receiving FDA de novo classification and U.S. market authorization for its ARC-EX System. This innovative device is the first non-invasive technology approved to improve hand strength and sensation in individuals with chronic SCI, offering hope to a community often told recovery is…
Read MoreEUR 3.7 million for ONWARD and its partners from the European Innovation Council (EIC)
The European Innovation Council Grant has been awarded to ONWARD and its research partners, including EPFL, IICT-BAS, CEA-Leti-Clinatec, and CEAList. The company also received the First Place Winner of the 2022 Brain-Computer Interface (BCI) Award. ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in…
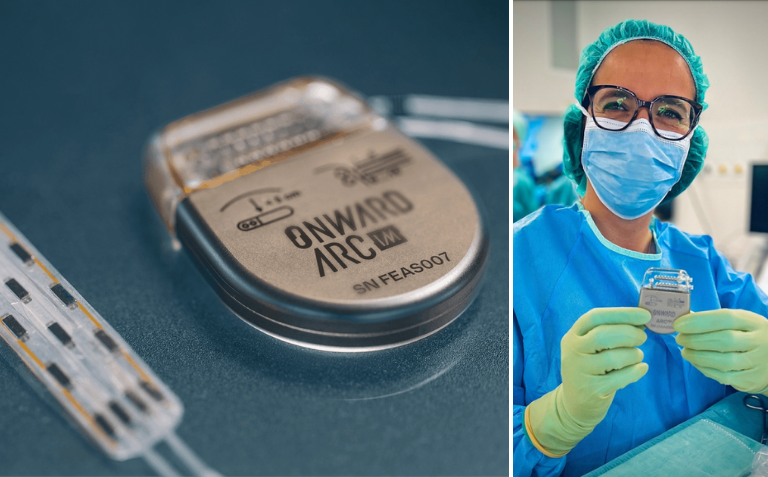
Read MoreONWARD’s Neurostimulator Restores Mobility for Patients with Spinal Cord Injury
This is the first time the ARCIM is used on a human patient. This implantable pulse generator (IPG) stimulates the spinal cord to restore movement and autonomic functions for people with spinal cord injury and other conditions that affect mobility. This first implant on a human patient is a major milestone in ONWARD’s successful…
Read More