Posts by Sandra Ansanay-Alex
Parkinson’s Disease: AC Immune Receives a Grant from The Michael J. Fox Foundation
A new USD 0.5 million grant brings up to USD 3.7 million the total amount The Michael J. Fox Foundation awarded the Swiss company. AC Immune SA, a Lausanne-based clinical-stage biopharmaceutical company pioneering precision medicine for neurodegenerative diseases, today announced that The Michael J. Fox Foundation for Parkinson’s Research (MJFF) awarded the Company a new…
Read MoreA wearable tissue health monitor
Sensawear wins a CHF 150,000 Venture Kick grant to develop its wearable tissue health monitor Sensawear has developed the world’s first truly wearable, textile-based, tissue oxygenation monitoring sensor using trusted, proven, non-invasive near-infrared spectroscopy. Specially integrated fiber optics will give users real-time feedback on deep tissue oxygen saturation without adding pressure against the skin.…
Read MoreSightIn Health wins a CHF 150,000 Venture Kick grant
The Swiss start-up wants to develop an AI-SaaS to democratize the use and interpretation of ultrasound SightIn Health’s Machine Vision and Artificial Intelligence software enables anyone to expertly operate, interpret, and analyze point-of-care ultrasound – without the need for any prior training. The real-time AI guidance system helps operators find the correct anatomical scanning…
Read MoreDayOne Tech Accelerator presents its first cohort
The DayOne Tech Accelerator in the Canton of Jura has just announced its winners. Three Medtech startups are starting a 12-month programme. Experts will help to push them to a higher level. CNS Therapy (Zug): Drug-free pain treatment The company has developed the ability to treat chronic pain patients using neuromodulation and behavioral therapy.…
Read MoreAC BioScience closes a 4th seed-round of CHF 2.5 million
AC BioScience, a Biopôle-based clinical-stage biotech company pioneering the development of novel therapies in immuno-oncology and tumour vascular normalization, announces a fourth Seed Round for CHF 2.5 million. Founded by Andreas Schlaepfer and Professor Christian Auclair, AC BioSciences develops personalized molecular therapies designed to optimize efficiency or bolster the therapeutic benefit for particular groups…
Read MoreAlcon buys Aerial Pharmaceutics for $770 million
Ophthalmic devices and Geneva-based Alcon has reached an agreement to acquire North Carolina-based Aerie Pharmaceuticals for $770 million (743.1 million francs). The transaction will strengthen the former Novartis subsidiary’s position in the fields of glaucoma and dry eye. Alcon will acquire Rocklatan (netarsudil and latanoproste) and Rhopressa (netarsudil), which are approved for the reduction…
Read MoreNovartis to list its subsidiary Sandoz on the stock exchange
Under consideration since 2021, the details of the demerger of Sandoz, the generic drug arm of Swiss pharmaceutical giant Novartis, are becoming clearer. It will not be sold, but listed on the Swiss and US stock exchanges in the second half of 2023. Sandoz will be the number one listed generic player in Europe. …
Read MoreTwiice’s exoskeleton allows paralysed people to walk again
Vaud-based and EPFL spin-off startup TWIICE receives a CHF 100 000 seed loan from FIT Every year, around 250,000 people suffer a spinal cord injury resulting in permanent paraplegia. The primary means of mobility for people with paraplegia is the wheelchair, which exposes users to long-term health problems such as loss of bone density,…
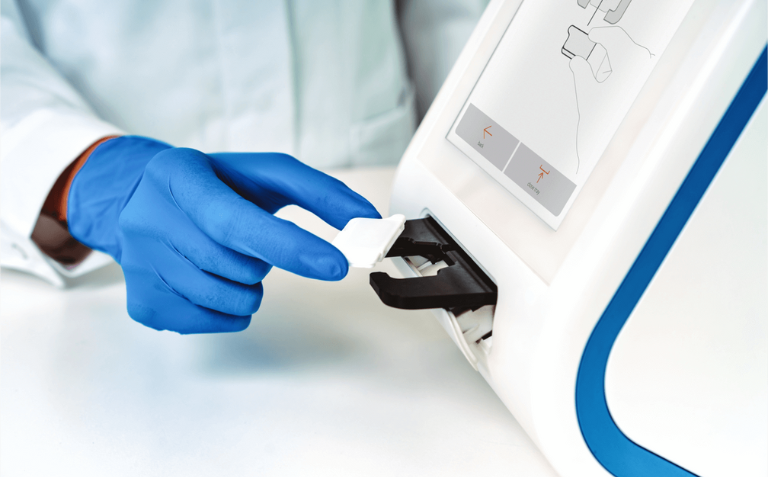
Read MoreAbionic’s test diagnoses sepsis 3 days before the standards methods
Abionic’s test identifies in 5 minutes the earliest signs of sepsis 3 days before the standard of care. The technology just received a European IVDR certification for its in-vitro diagnostics. Abionic SA, a developer of disruptive nanotechnology-based diagnostic solutions, announces that its Pancreatic Stone Protein (PSP) test on the abioSCOPE® has been certified by…
Read MoreObsEva plans job cuts
The ObsEva laboratory is to engage in a vast restructuring process in the face of difficulties encountered with the FDA with its drug in development Linzagolix. Job cuts might be on the agenda. The US Drug Administration (FDA) agreed in November 2021 to review the application for the drug to treat heavy periods associated with…
Read More